Abstract

Background: Currently, many infants with severe hemophilia A (HA) do not receive prophylaxis until at least one year of age, due to the challenges of factor (F)VIII administration. Emicizumab enables initiation of prophylaxis from HA diagnosis via subcutaneous administration, and may mitigate the risk of spontaneous and traumatic bleeding, including intracranial hemorrhages.
Aims: This interim analysis of HAVEN 7 (NCT04431726) aims to evaluate the efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) of emicizumab in infants with severe HA without FVIII inhibitors.
Methods: HAVEN 7 is a Phase IIIb, multi-center, open-label study of emicizumab in infants ≤12 months with severe HA without FVIII inhibitors. Informed consent from parents/legally authorized representatives and ethics approval have been obtained. Participants receive subcutaneous emicizumab 3mg/kg weekly for 4 weeks, then 3mg/kg every 2 weeks for 52 weeks; at Week 53, participants can continue with this regimen or switch to 1.5mg/kg weekly or 6mg/kg every 4 weeks for the 7-year long-term follow-up period. Efficacy endpoints include the number of treated bleeds; all bleeds; treated spontaneous bleeds; and treated joint bleeds. Annualized bleed rates (ABR) are estimated using a negative binomial regression model. Safety endpoints include adverse events (AEs); thromboembolic events (TEs) and thrombotic microangiopathies (TMAs); and immunogenicity (incidence of anti-drug [emicizumab] antibodies [ADAs]). PK and PD endpoints include plasma trough emicizumab concentration; FIX and FX antigen concentrations; and effect of emicizumab on activated partial thromboplastin time (aPTT), thrombin generation (TG) and FVIII-like activity (measured using a chromogenic assay with human factors).
Results: At the interim analysis cut-off date (March 31, 2022), 54 participants
(55.6% [n=30] ≥3-≤12 months old; 44.4% [n=24] <3 months) had ≥1 dose of emicizumab; all were male, with a median (range) age of 4.5 (0-11) months. Of these, 30 (55.6%) were minimally treated (≤5 exposure days with hemophilia-related treatments [i.e., FVIII, plasma, cryoprecipitate or whole blood products]) prior to the study, and 24 (44.4%) were previously untreated. Median (range) emicizumab treatment duration was 42.1 (1-60) weeks.
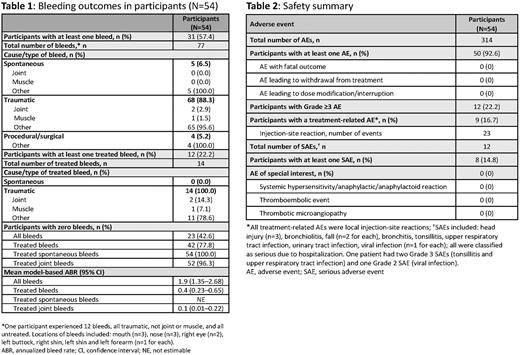
Overall, 77 bleeds were reported in 31 participants (57.4%): 88.3% traumatic; 5.2% procedural/surgical; 6.5% spontaneous (Table 1). One participant experienced 12 bleeds (all traumatic; none joint or muscle; none treated). In total, 14 treated bleeds, all traumatic, were reported in 12 participants (22.2%). No intracranial hemorrhage occurred and no participant experienced >2 treated bleeds.
Mean model-based ABR (95% confidence interval [CI]) for treated bleeds was 0.4 (0.23-0.65); it was 1.9 (95% CI: 1.35-2.68) and 0.1 (95% CI: 0.01-0.22) for all bleeds and treated joint bleeds, respectively (Table 1). Zero treated bleeds were reported in 77.8% of participants (n=42), while 42.6% of participants (n=23) had no bleeds at all.
Fifty participants (92.6%) had ≥1 AE and nine (16.7%) had ≥1 emicizumab-related AE (all injection-site reactions). No AE leading to treatment withdrawal/modification/interruption occurred (Table 2). Eight participants reported 12 serious AEs (SAEs); none was considered emicizumab related. There were no deaths, TEs or TMAs at the time of this interim analysis.
PK data were evaluable in 52 participants. Mean trough concentrations of emicizumab increased with loading doses, with concentrations of 63.2µg/mL (95% CI: 59.5-66.8) at Week 5; steady-state concentrations were maintained at 60-65µg/mL thereafter. Emicizumab concentrations were higher than those observed with the same dosing regimen in older people with HA in the HAVEN 1-4 studies (46.7 ± 14.9µg/mL). None of the 48 participants evaluable for immunogenicity analysis tested positive for ADAs.
Mean FVIII-like activity increased during loading to 21U/dL and was sustained thereafter. TG peak height increased during loading and continued to increase to reach 100nM from Week 17 onwards. aPTT was normalized by Week 3 (first data point post-baseline) in most participants. Mean FIX and FX concentrations were unaffected by emicizumab.
Conclusions: The results of this interim analysis indicate the efficacy and confirm the safety of emicizumab in infants with severe HA without FVIII inhibitors. PK and PD activities were sustained.
Disclosures
Pipe:HEMA Biologics: Consultancy; Bayer: Consultancy; Freeline: Consultancy; Takeda: Consultancy; Sangamo Therapeutics: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; ASC Therapeutics: Consultancy; Apcintex: Consultancy; Spark Therapeutics: Consultancy; CSL Behring: Consultancy; Sanofi: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy; Regeneron/Intellia: Consultancy; Novo Nordisk: Consultancy; UniQure: Consultancy. Collins:Roche/Genentech: Membership on an entity's Board of Directors or advisory committees. Dhalluin:Roche Pharmaceuticals: Current Employment. Kenet:BPL: Consultancy, Research Funding; Alnylam: Research Funding; Novo Nordisk: Consultancy, Honoraria; ASC Therapeutics: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy, Honoraria; Sanofi-Genzyme: Consultancy, Honoraria; Shire: Research Funding; Opko Biologics: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Roche: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; UniQure, SPark, Sobi, CSL: Honoraria. Schmitt:F. Hoffmann-La Roche Ltd: Current Employment, Current holder of stock options in a privately-held company. Buri:F. Hoffmann-La Roche AG: Current Employment, Current equity holder in publicly-traded company. Jiménez-Yuste:Roche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Octapharma, CSL Behring: Research Funding; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria. Peyvandi:Grifols: Honoraria; BioMarin: Honoraria; Roche: Honoraria; Takeda: Honoraria; Sobi: Honoraria; Sanofi: Honoraria. Young:Hema Biologics: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Spark: Consultancy, Research Funding, Speakers Bureau; Grifols: Research Funding; CSL Behring: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria; Genentech: Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria; LFB: Consultancy; Viatris: Patents & Royalties. Oldenburg:Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; University Clinic Bonn: Current Employment; Freeline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Sparks: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Biotest: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodation, expenses, Research Funding, Speakers Bureau. Mancuso:Bayer: Consultancy, Research Funding, Speakers Bureau; Biomarin: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; Kedrion: Consultancy, Speakers Bureau; LFB: Consultancy, Speakers Bureau; Octapharma: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau; Spark: Speakers Bureau; UniQure: Consultancy, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau. Kiialainen:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Chang:Spark Therapeutics: Current Employment; F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company. Lehle:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Fijnvandraat:EAHAD: Membership on an entity's Board of Directors or advisory committees; CSL Behring, Novo Nordisk, Sobi: Research Funding; Sobi, Sanofi, Novo Nordisk, Roche: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal